Date:2025-07-31 Views:0
Dental amalgam alloys are formed by the reaction of liquid mercury with a silver-based alloy powder. The powder compositions fall into two broad categories: low-copper and high-copper. Low-copper alloys contain more than 65 % silver, 29 % tin, and ≤ 6 % copper. High-copper alloys, developed during the 1960s and 1970s, contain up to about 30 % copper.
When silver–tin alloy powder contacts mercury, silver and tin dissolve in the mercury while mercury diffuses into the powder. Because the solubility of both silver and tin in mercury is limited, precipitates form: γ (Ag₂Hg₃) and, for the tin phase, γ₂ (Sn₈Hg). These reactions continue until the mercury is exhausted. The final composite consists of unreacted alloy particles surrounded by layers of silver–mercury and tin–mercury intermetallic compounds, with some residual porosity. Amalgam properties depend on the mercury-to-powder ratio, powder composition and morphology, and the condensation technique. High-copper alloy powders may be blends of silver–copper and silver–tin powders, or pre-alloyed silver–tin–copper powders. The basic amalgamation mechanism (interdiffusion and precipitation) is the same as for low-copper alloys.
In binary blends of silver–copper and silver–tin powders, a Cu₆Sn₅ layer forms on the surface of the silver–copper particles. This Cu₆Sn₅ phase results from tin that has dissolved and diffused to the silver–copper particle surface. This reaction eliminates most or all of the γ₂ (Sn₈Hg) phase, the least durable constituent of the amalgam and the phase most susceptible to corrosion in the oral environment.
When pre-alloyed silver–tin–copper powders are amalgamated, similar reactions occur, but the Cu₆Sn₅ phase adopts a different morphology—rod-like precipitates that coat the particles. This rod-like structure improves bonding to the matrix and increases resistance to deformation. The final amalgam consists of unreacted alloy particles coated with Cu₆Sn₅, embedded in a γ₁ (Ag₂Hg₃) matrix, thereby minimizing or eliminating the undesirable γ₂ phase.
The earliest powders used for dental restorations were lathe-cut powders produced by machining cast ingots. This process yields dense, slightly elongated chips (Figure 10-12). Before machining, the ingots are homogenized by heat treatment just below the alloy’s incipient melting point. The cooling rate at the end of homogenization can be adjusted to alter the phase distribution in the ingot.
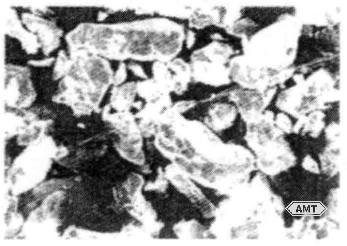
Figure 10-12 SEM micrograph (250×) of lathe-cut dental amalgam powder
After homogenization and machining, the powders may be further processed—for example, ball-milled to reduce particle size. Proprietary acid treatments can be used to wash the powders, modifying their amalgamation behavior. Stress-relief treatments may also be applied to eliminate residual stresses introduced during machining.
Atomized powders produced by conventional atomization are slightly irregular in shape (Figure 10-13); smaller particles tend toward sphericity. These powders are heat-treated to increase grain size within the particles, thereby slowing the reaction with mercury. Atomized powders are often acid-washed. Mixed blends of lathe-cut and atomized powders are also in use (Figure 10-14).
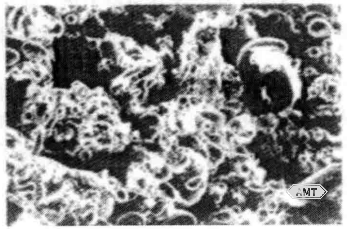
Figure 10-13 SEM micrograph (250×) of atomized dental amalgam powder
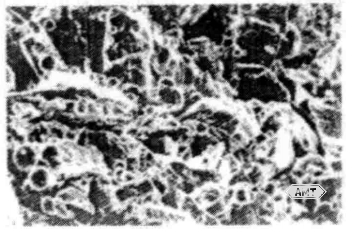
Figure 10-14 SEM micrograph (250×) of a blended mixture of lathe-cut and atomized amalgam powders [the spherical particles are atomized powder]
Key properties of dental amalgam include compressive strength, setting characteristics, and dimensional stability. Powder variables such as particle size distribution, surface area, and composition strongly influence these properties. Commercial powders typically have a particle size < 44 µm (–325 mesh) with an average of ~30 µm. Finer powders result in faster setting and higher early strength, but excessive ultrafine (< 3 µm) fractions increase surface area, requiring more mercury and potentially degrading properties.
Particle shape also plays a role. For a given size, spherical powders have lower surface area than lathe-cut powders, requiring less mercury—an advantage from a mechanical-property standpoint. Table 10-2 shows the relationship between compressive strength and residual mercury for lathe-cut-powder amalgam: higher mercury content markedly reduces compressive strength.
Table 10-2 Compression strength vs. residual mercury content of chip-powder amalgam
Residual Hg (%) | Compression strength | |
/MPa | /psi | |
52 | 57 | 8300 |
54 | 54 | 7800 |
56 | 43 | 6300 |
58 | 36 | 5200 |
Amalgams made from spherical powders exhibit greater plasticity than those from lathe-cut powders. Dentists can achieve higher strength with lower condensation pressures, but the increased plasticity requires care during carving to achieve the desired anatomy.
As noted, high-copper amalgams contain little or no γ₂ phase. Because γ₂ is weaker, more prone to creep, and less corrosion-resistant than γ, its absence is beneficial. Comparative studies show that high-copper materials possess higher initial and aged compressive strength but slightly lower creep resistance.
Some alloys contain zinc as a deoxidizer during melting. While zinc improves ductility, its presence can cause excessive expansion if moisture is present during trituration and condensation. This expansion is associated with hydrogen evolution and begins 4–5 days after placement, potentially causing pain. Low-zinc, high-copper alloys avoid this problem.
Leave your email for more ebooks and prices📫 !
Contact:Fidel
Tel:021-5512-8901
Mobile:19916725893
Email:sales7@atmsh.com
Address:No.398 Guiyang Road Yangpu China